May 1, 2025 9:00:00 AM
Microfluidics, an IDEX MPT company, is delighted to announce an exciting..
LNPs, including various lipid-based platforms such as liposomes, nanostructured lipid carriers (NLCs), and solid lipid nanoparticles (SLNs), are critical for the delivery of protein and amino acid-type antigens.
From R&D all the way up to production scale it is critical to use the best, most suitable methodology. Microfluidics is delighted to be partnering with Inside Therapeutics to extend our range of equipment solutions and help our customers to reach their LNP goals.
The challenge in LNP production is manufacturing lipid-based nanoparticles which exhibit strong efficacy and functionality, that not only can be repeated batch-to-batch but also be scaled from lab and/or pilot size to mass-production volumes.
Choosing the right methodology for LNP manufacturing is critical. Are you clear on which is most suitable method for your LNP formulation - a top-down or bottom-up approach? We are here to help you.
Microfluidics is delighted to add the TAMARA nanoparticle formulation system to the portfolio. An innovative platform that covers two R&D stages from screening to in-vivo scale in one machine, using microfluidic technology.
We are the only company that offers both the top-down as well as the bottom-up LNP manufacturing methodology. Our equipment is simple to use, gives reliable, superior results and the ability to scale.
Microfluidizer® technology is a trusted top-down solution for lipid nanoparticle production. The equipment is easy to operate and produces consistent particle sizes with narrow distributions.
The TAMARA platform adds R&D scale, bottom-up technology to the portfolio. The simple to use, plug & play module can process samples in under 2 minutes. Reusable microfluidic chips make it a sustainable choice.
Bringing TAMARA into our portfolio means that we can offer customers the ability to compare a top-down or bottom-up LNP manufacturing methodology.
Furthermore, we offer the ability to scale up to production volumes and help deliver your product to the market.
Our dedicated team of application engineers & subject matter experts offer support throughout your journey.
The most common nanoparticle formulation method relies on the fast mixing of two miscible phases (lipids/polymers in an organic solvent and aqueous buffer), leading to a drop in solubility of the lipids/polymers, which start to agglomerate to form nanoparticles. Commonly known as nanoprecipitation/self-assembly process.
Mixing speed affects the nanoparticle size - faster mixing produces smaller particles. Also, the more efficient and homogeneous the mixing is, the better the control over the size and distribution.
Microfluidic chip-based platforms can maintain the fluid flow in laminar conditions, and provide optimal control of the final nanoparticle physicochemical parameters.
.webp?width=252&height=155&name=TAMARA-11-2024-inside-therapeutics-800-800x494%20(1).webp)
Benefits:
The phospholipid, carrier oil and any hydrophobic actives are dissolved in a solvent, which is subsequently removed via evaporation. A buffer is added to the resulting precipitate and then warmed and vortexed to hydrate the phospholipids.
The antigen (or other hydrophilic actives) are added to the solution whereupon large multi-layer vesicles (MLVs) are generated.
These MLVs are then processed through a Microfluidizer® processor to reduce their size to small uni-lamellar vesicles.
Benefits:
For more information, view our application note on creating RNA vaccine delivery systems.
This application note compares the in-situ encapsulation method to the post adsorption approach of LNP manufacturing. It is concluded that the post absorption approach is highly suitable for large-scale manufacturing & responding to pandemic situations.

Our application experts are happy to discuss your project and help you select the ideal equipment that will ensure your drug development success. Fill out your details and we will be in touch.
Microfluidizer® technology has been proven as a seamless path for scaling up LNP production, enabling a pharmaceutical company successfully create the ideal liposomes to treat carcinomas.
Small and Uniform Particles
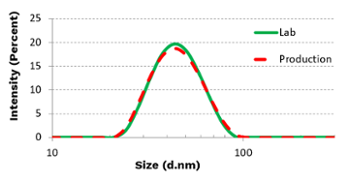
The average particle size was reduced to around 40 nm. The polydispersity index (PdI) was <0.1, indicating high particle size uniformity.
Process Scalability
The process was scaled up directly from lab scale to the production scale with results obtained from the production machine almost identical to the results produced by the lab machine (see graph).
When doing the initial research steps, it is important to get the screening right. TAMARA provides control over particle size, Polydispersity index (PDI), and Encapsulation efficiency (EE%).
Read the Application Note to explore how to streamline your screening methods.

Recommended downloads for manufacturers looking to achieve stable nanoemulsions.