Nanoemulsions have been proven to offer significant benefits as drug delivery systems. Biodegradable oil-in-water emulsions have surfaced as being effective vaccine adjuvants because they enhance the tolerability and have shown increased immune response without the need of an additional immune stimulator.
Most adjuvanted vaccines are administered to the patient as an injection. This means that sterilization becomes a critical step. For systems with heat-labile substances, terminal sterilization using heat is not always a viable option, so sterile filtration is deployed instead.
Validation of the sterile filtration step can be challenging and requires a robust solution with combined process development and filtration technology. Firstly, sterile filtration is done by passing product through 0.22 µm rated sterilizing-grade filters. Any particles present that are greater than 220 nm can cause significant reduction in the filter throughput or eventually clog the filters. Therefore, the manufacturing process needs to produce adjuvant nanoemulsions with not only a small particle size but also tight particle size distributions. Secondly, various sources of contamination, introduced during the manufacturing processes and environment, can create additional challenges for the sterile filtration step. Finally, the nature of the adjuvant nanoemulsions makes them high risk fluids with potential reduced bacterial retention efficiency and losing product sterility.
In this blog post, we will demonstrate the production method and successful sterile filtration process of a nanoemulsion based adjuvant.
Sterile Filtration
If a product is not processed in an aseptic environment (which can be expensive and difficult to maintain), contaminants, including bacteria, may be introduced from various external sources, from the manufacturing process or during maintenance.
To combat this, sterilizing grade filters can be used to discard these contaminants through both removal and retention mechanisms. As per the US FDA’s Guidance on Sterile Drug Products Produced by Aseptic Processing released in 2004, the sterilizing grade filter was defined as, “A filter that, when appropriately validated, will remove all microorganisms from a fluid stream, producing a sterile effluent” [ref 1]. These filters typically have a mean pore diameter around 0.22 µm. It is important to note that while these filters are rated to have 0.22 µm pore size, the actual membrane can have many pore openings that are much larger than 0.22 µm, which would allow bacteria to penetrate some distance into the membrane depth.
This same guidance also points out that validation of sterilizing filters “should include microbiological challenges to simulate worse-case production conditions for the material to be filtered and integrity test results of the filters used for the study.” A commonly used micro-organism is Brevundimonas diminuta and a challenge concentration of at least 107 CFU per cm2 of effective filtration area should generally be used. Pre-filtration is also usually required for emulsions to help achieve consistent bacteria reduction and subsequent bacterial retention studies.
Microfluidizer® Technology
Microfluidizer® processors utilize high pressure to pump multi-phase fluids through microchannels located in a fixed geometry Interaction ChamberTM. At high pressures, the fluid velocity within the microchannel can reach higher than 400 m/s, resulting in shear rates of up to 108 s-1. These high shear rates and intense micro-mixing generated by subsequent turbulent energy dissipations are capable of significantly and efficiently reducing particle/droplet size. Not only is the desired size of the particles achieved, but the particle size distribution produced is also very narrow.
As shown below, scaling up while utilizing Microfluidizer® technology is quite simple. While lab scale units utilize single microchannel Interaction Chambers™, production scale units place these microchannels in parallel, which allows for a higher throughput, and at the same time, all material still faces the same levels of shear, thus guaranteeing scale-up results.
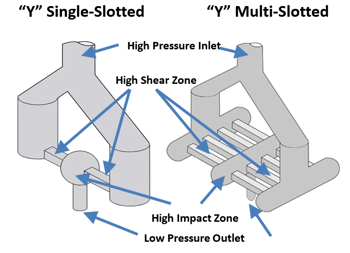
Case Study Methodology
In this case study, a vaccine adjuvant was created using a Microfluidizer® processor, which was then passed through sterilizing grade filters manufactured by Pall Life Sciences. The entire process is illustrated in the diagram below [Ref 2]. The model adjuvant emulsion is formulated with squalane oil. This formulation was processed under various conditions through a pilot scale Microfluidizer® processor in order to create nanoemulsions with different particle size characteristics. The nanoemulsions were first pre-filtered with a Pall Supor® EKV filter and then filtered through a Pall Fluorodyne® EX EDF filter, both of which are sterilizing grade filters. The sterile effluent was then challenged according to the bacterial retention study mentioned above for process validation.
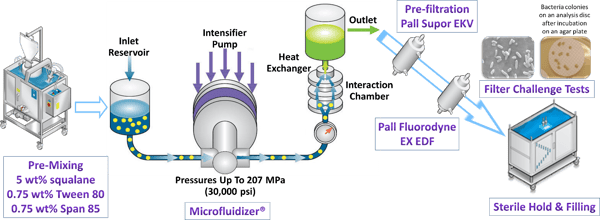
Results
Vaccine Adjuvant Nanoemulsion Production
All the different nanoemulsion models (which were created with various particle size characteristics as a result of different processing conditions) were monitored and the results are given below:
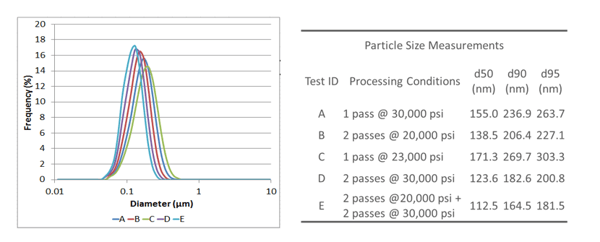
Particle size measurements showed that the Microfluidizer® processor is not only very efficient in creating nanoemulsions with small droplet sizes, but that it can also be easily tuned to achieve desired outcomes, e.g. nanoemulsions with both specific, and different, mean particle sizes. All tests were able to achieve a d50 particle size well below 200 nm (or 0.2 µm), ranging from around 110 nm to 170 nm. d95 measurements ranged from as small as 180 nm to 300 nm. All the nanoemulsion samples created had very tight particle size distributions.
Sterile Filtration
Nanoemulsions with d50 particle sizes of 155 nm (Sample A), 134 nm (Sample B), and 124 nm (Sample D) were passed through the Pall Fluorodyne EX EDF filter. The resulting throughput for each sample is shown below:
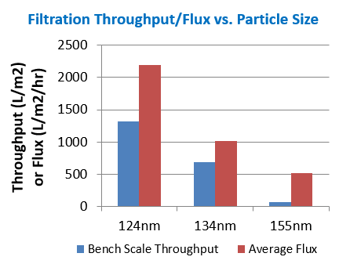
Whilst the d50 particle size for each sample would suggest that they should be able to be processed through a 0.22 µm filter, the actual throughput observed and the flux results achieved varied greatly between these nanoemulsion samples. The nanoemulsion with a d50 particle size of 124 nm was able to be passed through the filter much more quickly than the nanoemulsions with slightly larger d50 particle sizes of 134 nm and 155 nm, therefore achieving much higher throughputs - almost a 20 times higher throughput when comparing the nanoemulsions with d50 of 124 nm and 155 nm. This proved that despite having a d50 particle size in the 'correct' range, the entire particle size distribution must be taken into consideration when attempting to efficiently process samples through a sterile filter. The higher throughputs in this case study are directly related to lower d95 values, indicating that small, tight particle size distributions are desired in order to efficiently sterile filter products.
In reality small, tight particle size distributions could mean a huge saving on the amount spent on filters. The fewer the number of larger particles means that fewer particles will get caught in the membrane pores, thus both increasing throughput and reducing the amount of clogged filters. As a result, a much smaller filter area, or fewer number of filters may be required to process the same amount of product. These particle size results can be achieved while using a Microfluidizer® processor by precisely choosing the correct processing conditions including Interaction ChamberTM, pressure, and number of passes.
In the study, particle size was also measured both pre and post-filtration (while being filtered under various pressures) for a nanoemulsion sample with the 124 nm d50 particle size. As shown in the figure below, neither the filtration process, nor the selected filter membrane materials affected particle size, as d10, d50, and d90 particle sizes remain essentially unchanged from their pre-filtration size (blue bars) whether being filtered at 30 psi (red bars) or 60 psi (green bars). This indicates that the integrity of the nanoemulsion was not significantly altered.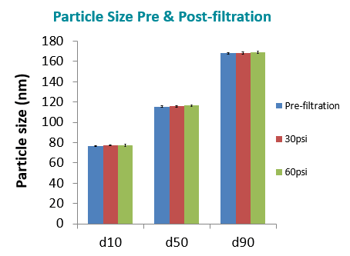
Bacterial Challenge Test
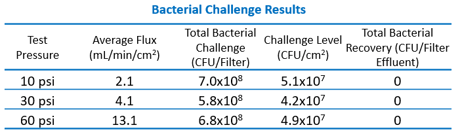
The nanoemulsion with 124 nm d50 particle size and the EDF filters were selected for the bacterial challenge test. This is an important validation step to determine if the nanoemulsion or process conditions influence the challenge organism or the filter membrane, which would lead to failure to retain the test organism. The results are shown in the table below. All filters indicated complete bacterial retention, which is zero bacteria in the effluent, at all filtration pressures tested. This indicates that both the adjuvant nanoemulsions and their production process, as well as the sterile filtration process is successfully validated.
Summary
To summarize, the research showed that nanoemulsion particle size and distributions can be precisely controlled by adjusting processing parameters on the Microfluidizer® processor. These precise sizes and distributions are necessary to ensure efficient processing through a sterile grade filter, without loss of throughput or filtering capability.
Furthermore, an effective sterile filter should not drastically affect particle size, meaning that the product you put in to the filter should be essentially the same as the product you get out – thereby ensuring product quality.
You can learn more about Microfluidics Equipment and Technology for Vaccine Development and Production here or download our infographic on terminal sterilization of nanoemulsion adjuvants using the button below.
Ref 1: US Food and Drug Administration. Guidance for industry. Sterile drug products produced by aseptic processing: good manufacturing practice. Rockville, MD. Sep, 2004.


