According to the World Health Organization (WHO), pneumonia is the leading cause of death in children under 5 globally resulting in almost a million child deaths annually.1 Due to undeveloped immune systems, children are more receptive to bacteria and viruses which target their immature systems, leaving them vulnerable. Also due to scarce healthcare access, pneumonia deaths are greater in low income countries than in middle to high income countries. Therefore, developing an effective pneumococcal conjugate vaccine (PCV), that is both accessible and cost efficient to third world countries, is critical in lowering deaths globally among poor and vulnerable populations. The Serum Institute's recent PCV launch of Pneumosil, in India, is essential in impacting this global immunity inequity problem.
In the development of PCVs, a critical inverse relationship has been found between the molecular weight of polysaccharides utilized and the content of free polysaccharides in solution. This fact is of key importance in enhancing immunogenicity. Microfluidizer® technology has been instrumental in the development of highly efficient conjugate pneumococcal vaccines with its ability to lower the content of free polysaccharides in solution and thereby contributing in the production of an elite vaccine of enhanced stability, sterilization, and immunogenicity.
Polysaccharide conjugate vaccines are vaccines that work by conjugating a bacterial polysaccharide antigen to a carrier protein. This process results in heightened immunity by promoting activation of the immune response. A challenge, with polysaccharides and their often high molecular weights, is that it can create a viscous solution, resulting in sterile filtration problems later in the processing system. In addition, it can make isolation of the conjugated polysaccharide difficult due to interference from unbound free polysaccharides inhibiting the immunogenicity of the vaccine.
Reducing the molecular weight prior to conjugation is imperative in ensuring the product is more manageable and creates a more sterile process resulting in fewer disruptions, better quality, and enhanced immunogenicity. In addressing the complexities of pneumococcal vaccine development, the impact of molecular weight reduction, in polysaccharides, is critical for uniformity and stability.
Traditionally, molecular weight reduction was achieved chemically but risked modifying the polysaccharide structure and often resulted in polydisperse polymers. As an alternative, molecular weight can be more effectively reduced by applying high shear force which does not alter the polysaccharide structure and results in a more homogeneous final product with low polydisperse polymers. Microfluidizer® technology allows for better purification and reduced viscosity.
Microfluidizer® Technology and How It Works
Microfluidizer® technology is instrumental in helping vaccine manufacturers overcome the challenges of reducing molecular weight of pneumococcal polysaccharides. By adjusting the number of passes through high shear pressure, a more uniform and stable result is produced.
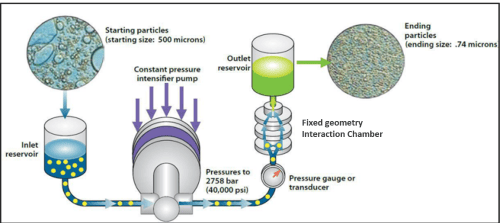
The Microfluidizer® Processor’s superior technology is the result of incorporating the revolutionary Interaction Chamber™. By combining high impact and shear force through micro-channel walls, Microfluidics has superior results over its competition. The Interaction Chamber™ allows consistent quality with scale up capabilities. This expertise differentiates itself not only by providing repeatable results but also duplication from lab to pilot to manufacturing.

- Consistently higher shear rates
- Repeatable results batch-to-batch, regardless of volume
- Particle size reduction with uniform results and with fewer passes
- A final product with greater stability and longer shelf life
Microfluidics is proud to be partners in creating solutions to global health issues through superior technology, commitment to excellence and unrivaled results. In working to create effective vaccines, Microfluidics is a leader in providing revolutionary technology which can be scaled up from lab to manufacturing. Microfluidics has a proven track record in creating consistent repeatable results, batch-to-batch while ensuring the same uniformity with less disruption and stable results. From our engineers to our technical support, Microfluidics is committed to leading the industry in providing superior knowledge to achieve superior results.
For more information, download the application notes.
References
1World Health Organization – who.int
Whang, Yoon Hee, "Reduction of free polysaccharide contamination in the production of a 15-valent pneumococcal conjugate vaccine,journals.plos.org
Posted by
Matt Baumber
